Abstract
Olanzapine has been shown to significantly decrease nausea and emetic episodes associated with chemotherapy in patients undergoing hematopoietic stem cell transplant (Monson, Greer, Kreikemeier, & Liewer, 2020). Additionally, Olanzapine has been shown to improve clinical outcomes in autologous stem cell transplant patients when added to triplet anti-emetic therapy of ondansetron, fosaprepitant and dexamethasone without any negative effects on time to engraftment (Clemmons, et al., 2018). Although this study did not show any delay in time to engraftment, there is only a small amount of data looking at this question, including Clemmons et al. (2016) and Trifilio et al. (2017). Other studies (Navari et al., 2016) demonstrated a decrease in chemotherapy related nausea and vomiting in patients receiving Olanzapine, but did not provide data on engraftment times as this was in standard chemotherapy recipients.
At Colorado Blood Cancer Institute (CBCI), Olanzapine was used for a period of time for anti-emetic prophylaxis both pre and post autologous stem cell transplant. There was concern regarding whether or not Olanzapine was causing delays in time to engraftment or even potentially graft failure and use of the drug in transplant anti-emetic regimens was discontinued.
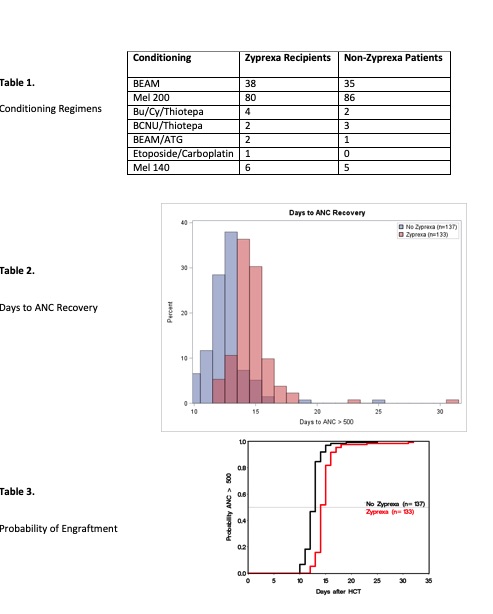
We hypothesized that Olanzapine does not cause higher incidence of graft failure, as compared to patients who do not receive Olanzapine as part of their anti-emetic regimen during the pre and post autologous stem cell transplant period. A retrospective analysis (n = 272) was conducted on patients who underwent autologous stem cell transplant between 2019 and 2020. 134 of these patients received Olanzapine during conditioning and up until time of engraftment, and 138 patients had no Olanzapine exposure throughout their conditioning and transplant. Conditioning regimens were equal between the two groups (Table 1). The average number of days on Olanzapine was 10.7, and the average dose was 7.5mg daily. For the purposes of this study engraftment was defined as the first day post stem cell infusion that the patient had an absolute neutrophil count (ANC) of greater than or equal to 500.
Findings showed that the mean day of engraftment in the patients with Olanzapine exposure was 14.69; in the non-Olanzapine group, the mean day of engraftment was 12.64 (Table 2). Using the two-sample t-test, this difference (2.05 days, 95% CI (1.60-2.51)) is significant (p<.0001).
Based on our findings, there is evidence to support Olanzapine causing a slightly delayed time to engraftment in autologous stem cell transplant patients, but not a higher incidence of graft failure, although a randomized controlled trial would be needed to fully investigate. There is clear data showing increased ability to manage chemo therapy induced nausea and vomiting. While a randomized controlled trial would be needed to fully investigate, given there is not a proven increase in graft failure, Olanzapine should be considered as a desirable option to treat and prevent chemotherapy induced nausea and vomiting in autologous stem cell transplant patients.
No relevant conflicts of interest to declare.
Olanzapine (Zyprexa) - Used off-label for the treatment and prevention of chemotherapy induced nausea and vomiting

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal